INTRODUCTION
Using of bio-pesticides developed on the basis of micro-organisms or products of their vital activity in modern plant protection technologies is constantly increasing. Depending on the mechanism of action, different microbial preparations are specialized against specific pathogens. Widely used, especially in the greenhouse vegetables are bio-fungicides: Granular Fuzaklin (Fusarium oxisporum var. licopersici); Trihodermin (Trichoderma sp); Poliverzum (Pythium oligandrum) and other. They show a good effect against a number of soil pathogens [2; 4; 6; 12; 14]. Much of microbial preparations have besides direct and indirect effects against pathogens as a result of antagonistic and competitive relations for space and nutrients that occur in microbial communities. The importation of a heterogeneous population of non-pathogenic microorganisms after only a few generations induces dominance over pathogenic and leads to a reduction of their density. Release of biologically active substances in the rhizosphere boost to growth and strengthen the overall plant health [11]. Most of the pathogenic microorganisms to plants live and grow in the soil microcoenosis together with a huge number of their antagonist microorganisms. With a strong antagonistic action against fungal pathogens are different species bacteria from genera: Bacillus, Pseudomonas, Agrobacterium, Streptomyces [8]. They are producers of numerous and various biologically active substances (enzymes, oligopeptides, aminoglycosides, antibiotics, etc.) which can be used for the isolation and establishment of new microbiological preparations completely safe to the environment [18]. The studies at those aspects are few and are not found in the newer literature for the tobacco [3; 7; 8; 9].
The purpose of this study is to make a screening of soil actinomycetes against pathogens of the genus Alternaria by tobacco and to establish the extent of their antagonistic action.
MATERIALS AND METHODS
The study was conducted over a period of two years. Laboratory «in vitro» and pot experiments have been made. The primary isolation of actinomycetes was carried out by plating diluted soil suspensions on various culture media, by the method of Koch. For the starting material were selected colonies, which were show antagonistic action against colonies of microscopic fungi. Insulations and rе-insulations of them (different numbers for different models — actinomycetes), on Gauze1 agar were made and they were input in pure culture. The test pathogen (Alternaria spp.) was isolated from diseased tobacco leaves, on Czapek agar and brought to a pure culture through several re-insulations. The isolated strains of actinomycetes were been stored on a slant Gauze1 agar at temperature 4C0. Their recovery was been accomplished by transferring at material (from air-mycelium) in a liquid medium of Gauze1 and culturing at 28C0, for 72h, in the induction of growth of the strain. Purity of microbial cultures was verified through superficial culture by exhausting method of Drigalskij [5]. The recovery and inspection of purity of the test-pathogen (Alternaria spp.) was conducted by the same procedure on Czapek agar.
Experiments «in vitro»
Testing of microbes antagonists — by the method of «test-wells» on agar-Czapek medium by 24-h culture of the pathogen. In each petri-dish has five “test-wells“.The antagonistic effect has been determined by metering the size of the sterile zone [3]. They were made of five replications. The isolation, identification, testing of microorganisms with antagonistic activity and of the pathogen was performed by classical methods accepted in microbiology and phytopathology [1; 3].
Amplification of strains Actinomycetes with the strongest antagonistic effect was performed by preparing inoculum of the appropriate strain. Transferring the mycelium and spores in 5 ml of sterile saline 0.9%, to give a cloudy suspension. The suspensions were introduced into 250 ml Erlenmeyer flasks with liquid media of Gauze 1 — 200 ml. They were cultured for 14 days at temperature 28C0 in Shuttle’s apparatus. Multiplication of the test-pathogen (Alternaria spp.) was conducted by the same procedure on Czapek agar.
Morphological characterization of actinomycetes cultures
With the inoculums of actinomycetes strains were made streaks-cultures on solid nutrient media: starch-ammonia agar and oat-agar. They are some of accepted for standards on International Streptomyces project identification (ISP). Mаcrо—morphological characteristic and micro-morphological feature of sporangiophore hyphae of 14 day cultures was made [15; 16; 17]. The characteristic of sporangiophore hyphae is performed by microscopy of roof glass-plate installed into the nutrient media after culture. The color of the aerial and substrate mycelium were determined by the color scale by Tresner-Backus with using key for grouping by Nonomura [10].
Pot experiments
With inoculums from the respective strains were made pot experiments: In containers with non-sterile soil (2kg) sown with tobacco seeds for growing seedlings, inserted inside inoculums of relevant strains antagonists and inoculum of the test-pathogen (Alternaria spp.). Quantities inocula were 150 ml per container. Pot experiments were performed in three replications. Containers with the inoculum of the test-pathogen (Alternaria spp.), and a liquid nutrient media of Gauze 1, to serve for the control. In infected soil were determined quantities of actinomycetes, the total amount of microscopic fungi and quantity of representatives of the genus Alternaria.
Analyses were performed by plating of dilute soil suspensions on starch-ammonia agar for actinomycetes and on Czapek agar for microscopic fungi, by the method of Koch in three replications. They were made in dynamics. Quantities were counted before to inoculation on 0 day, and on 3 day, on 7 day, on 15 day and on 30 day after inoculation, and were calculated as most probable number of cells per g of absolutely dry soil (MPN cells/ g a. d. s.). Belonging at the colonies to genus Alternaria was determined microscopically by the presence of typical for a genus conidiospores.
RESULTS AND DISCUSSION
The following scale for «in vitro» test of antagonist effect of actinomycetes against Alternaria spp. was adopted: 0-10mm no or negligible effect (-/+); 10-20mm weak effect (++); strong fungicidal effect from 20 to 30mm (+++) and more than 30mm — many strong fungicidal effect (++++). In the first year of the study were isolated and tested 40 strains of actinomycetes. Ten strains from these, had been demonstrated strong and very strong fungicidal effect (figure 1). They were stored and in the next year nine were revitalized, by the method described above. Three strains, only retain a very strong fungicidal effect — over 30mm sterile area. These are numbers strains: — SA 05; CzD 06 and Ash 012. With them were made pot experiments and mаcrо- and micro-morphological characteristics for identification to genus. The results of the laboratory test are shown in Table 1.
Table 1
Fungicidal effect against pure cultures of test-pathogen Alternaria spp. — results of «in vitro» experiments
| number strains | І тест | ІІ тест |
| SA 05 originally isolated from: starch-ammonia agar | 32-36mm ++++ | 35-39mm ++++ |
| CzD 06 originally isolated from: Czapek-Dox agar | 38-42 mm ++++ | 43-48mm ++++ |
| Ash 012 originally isolated from: Ashby agar | 36-40 mm ++++ | 38-45mm ++++ |
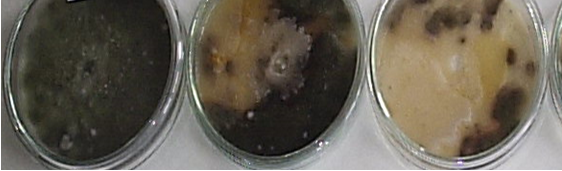
Fig.1. Antagonist effect of actinomycetes against Alternaria spp.
Makro— morphological characterization of the colonies of isolates and mikro-morphological feature of the sporangiophore hyphae showed, that the type of sporangiophores at all three strains were simple and according to the identification of key by Nonomura, the strains refer to the genus Streptomyces (table 2; figure 2). Analyses to determine their physiological-biochemical characteristics for species identification are required.
Table 2
Makro-morphological and micro-morfological characteristics of isolates, on starch-ammonia agar (SAA) and oat agar (OA), by 14-day microbial culture (by ISP)
| number strains | Makro-morphological characteristics of the colonies | mikro-morphological feature of the sporangiophore hyphae | ||
| Color on SAA | Color on OA | on SAA | on OA | |
| CzD06 | Aerial mycelium – dark gray
Substrate mycelium – dark gray Separation of pigment into the substrate — beige brown |
Aerial mycelium – gray
Substrate mycelium – dark beige Separation of pigment into the substrate — no |
Sporangiophore — Straight, slightly twisted
Hypal network — loose Section — RF (straight to curved) |
Sporangiophore — Straight, slightly twisted
Hypal network – loose. Numerous scattered spores Section — RF (straight to curved) |
| Ash012 | Aerial mycelium – light blue
Substrate mycelium – ivory color Separation of pigment into the substrate — no
|
Aerial mycelium – gray-blue
Substrate mycelium – yellowish Separation of pigment into the substrate — no |
Sporangiophore
Spiral by 1 step of semi-circles. Rudiments of nodules formation Section — S (spiral) |
Sporangiophore are highly the helical, with nodule formation.
Section — S (spiral) |
| SA 05
|
Aerial mycelium – white
Substrate mycelium – dark-wine Separation of pigment into the substrate — violet |
Aerial mycelium – light grey
Substrate mycelium – violet Separation of pigment into the substrate — violet |
Sporangiophore
Straight with 2-3 steps curves. Net structure. Section — RF (straight to curved) |
Sporangiophore
Straight with 3-4 steps curves. Net structure. Section — RF (straight to curved) |
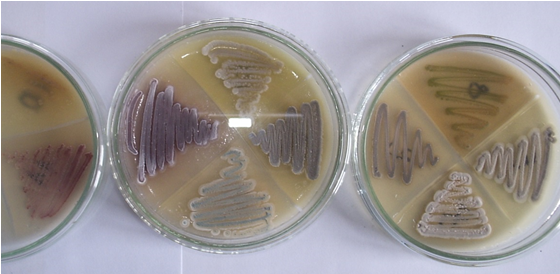
Fig. 2. Pure cultures of strains of Streptomycetes
The results from the pot experiments show a strong increase in the density of actinomycetes in the soil relative to control, at the three strains Streptomyces after introduction of the inocula. The increase was registered after 3rd day with maximum values around 15th day. The highest density was in variant with the introduction of inoculum by strain Ash 012. The differences with control were not statistically proven (Figure 3).
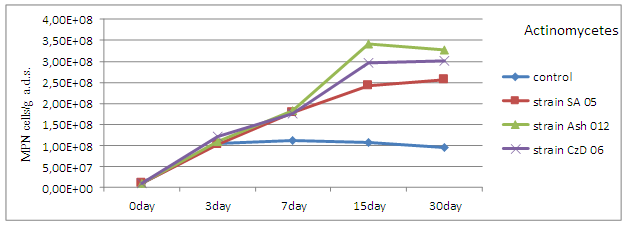
Fig. 3. Population density of actinomycetes (MPN cells/g a.d.s.) in the soil after the introduction of inoculums by strains Streptomyces.
The total quantities of microscopic fungi were decreased after the introduction of inoculums by strains Streptomyces. A very strong decrease was recorded using CzD 06 strain (Fig. 4). Differences with control are statistically proven with 95% confidence (t exp.SA05=2.360; t exp.Ash12=3.049; t exp.CzD06=3.229≥ t5%=2=306).
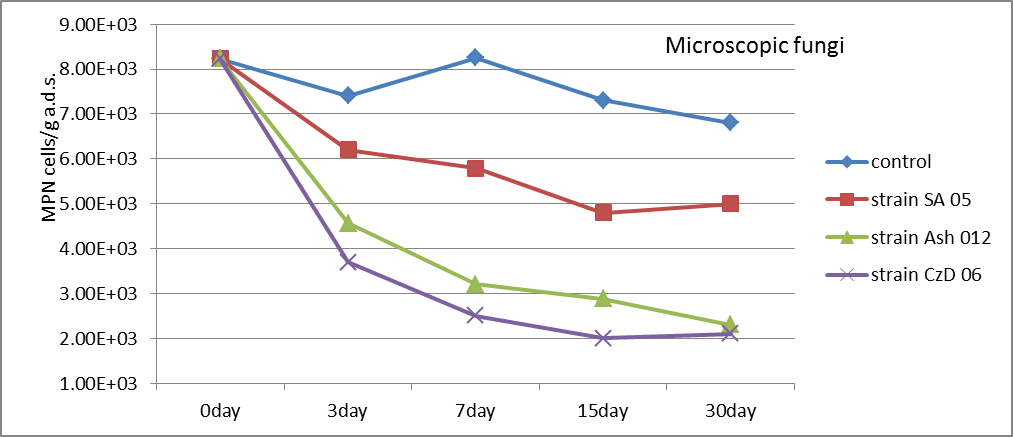
Fig. 4. Population density of microscopic fungi (MPN cells/g a.d.s.) in the soil after the introduction of inoculums by strains Streptomyces.
The density of the representatives of genus Alternaria spp., expressed as % of total numbers of microscopic fungi found at the relevant time reduced. The decrease compared to the control is obvious from 7th day and continued to increase until the end of the study period (Fig. 5).
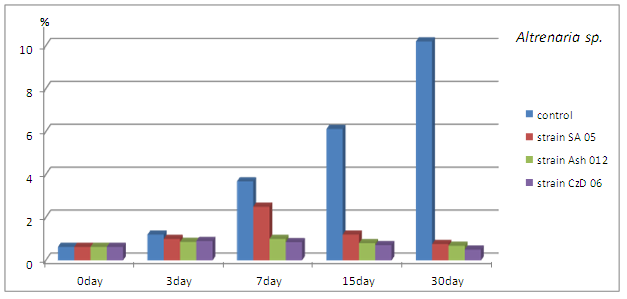
Fig. 5. Quantity of representatives of genus Alternaria expressed in % of the total amount of microscopic fungi
CONCLUSIONS
- From screened isolated from soil actinomycetes with extended very strong fungicidal effect against pathogen Alternaria spp., under conditions «in vitro»were three isolates: CzD 06; Ash 012 and SA 05.
- Antagonistic effect of strains actinomycetes against soil fungi, and in particular against representatives of the pathogens Alternaria was confirmed by the results obtained from pot experiments and the effect was reducing their density
- Macro— and micro— morphological characteristics of the strains, allow they to be identified to genus Streptomyces, according to standard taxonomic scheme and nomenclature adopted by the International Streptomyces Project — ISP (1980
REFERENCE
- Barnett H. L., Hunter B. B. (1998). Illustrated Genera of Imperfect Fungi. APS Press: St. Paul, MN, 1998: 7-196
- Bogatsevska N. et al. (2008). Manual for integrated pest management in vegetable crops. MAF Sofia, 2008: 253
- Gushterov G., Panayotov H., Todorov T. Vlahov S. Producing antibiotic substances from actinomycetes — antagonists isolated from soils in Bulgaria. 3rd Congress of Microbiology, Sofia, 2008: 73-77
- Georgieva O. & Georgiev G. Application possibilities of Bio-preparation “Trihodermin” in hydroponic growing tomatoes Proc. of 5th Scientific and Technical Conference «Ecology and Health», Plovdiv, 2004: 249-256
- Grudeva C., Moncheva P., Naumova S., Gocheva B., Nedeva T., Antonova-Nikolova S. Manual Microbiology. University ed. «St. Kliment Ohridski Sofia. 2006: 146-171
- Hoagland R. E. Microbial allelochemicals and pathogens as bio-herbicidal agents. Weed Technology v. 15, 2001: 835-857
- Hristeva Ts. & Bozukov H. Studying biological activity of soil Streptomycetes strains on the causes of brown leaf spots and wild fire on tobacco Proc. of the 2nd Balkan conference of tobacco, Plovdiv, 2002: 180-183
- Ivancheva-Gabrovska T. Vlahov S. Antibiotic substances against tobacco mosaic virus and tomato bronzovost in tobacco. 3rd Congress of Microbiology, Sofia, 1973: 279-283
- Kuzmanova J., Mollov Possibilities for the application of anti-phytopathogenic microorganisms. Proc. of the Scientific Session USB vol.1, 1998: 239-242
- Locci. Streptomycetes and related genera. (eds.) Williams S.T., Sharpe M.E., Holt J.G. Bergey’s Manual of Systematic Bacteriology, W. & W., Baltimore, 1989: 2451–2493
- Mayernik J. W. Microorganisms and there influence by diseases on the root-system: Using of Biolife by biological fungicide, Agricultural University of New Mexico, Tucumcari, 2002: 1-5
- Masheva St., Iankova C. Bioproducts control of diseases and pests in vegetable crops. J. New knowledge 3, 2012: 12-24
- Nonomura H. New for Classification and Identification of 458 species of the Streptomycetes included in ISP. J. Ferment. Technol. vol.52, 2, 1974: 78-92
- Sidiqui I. A., Qureschi S. A., Sultana V., Ehteshamul-Haque S., Gaffar A. Biological control of root rot-root knot disease complex of tomato. Plant and Soil, 227 (1-2), 2000: 163-169
- Shirling E. & Gottlieb D. Methods for species characterization of streptomycets (actynomycets). Int. J. System. Bacteriol. vol.15, 3, 1966: 313-340
- Shirling E. & Gottlieb D. Cooperative Discription of Type cultures of Streptomyces. II. Species discriptions from first study. Int. J. System. Bacteriol. 18, 2, 1968: 69-189
- Shirling E. & Gottlieb D. Cooperative Discription of Type straints of Streptomyces. Int. J. System. Bacteriol. vol. 18, 2, 1972: 69-189
- Vlahov S. & Antonova S. Origin of proteolytic enzymes in some soils Annual of Sofia University, vol. 3, part 78/79, 1990: 33-37[schema type=»book» name=»SCREENING OF SOIL ACTINOMYCETES WITH ANTAGONISTIC ACTIVITY AGAINST PATHOGENS OF THE GENUS ALTERNARIA AT TOBACCO PLANTS» description=»Antagonistic activity of 40 strains of soil actinomycetes against pathogens of the genus Alternaria at tobacco plants have been isolated and tested. Classical methods accepted in microbiology and phytopathology have been used. Three of them had retained a very strong fungicidal effect against the pathogen at «in vitro» experiments for two years. According to their macro- and micro- morphological characteristics they relate to the genus Streptomyces. Inoculums prepared therefrom were introduced into the soil and increased the total population density of actinomycetes and reduced the overall density of the microscopic fungi and pathogens of the genus Alternaria. These strains can serve as the basis to develop bio-fungicides.» author=»Hristeva Tsveta Hristova» publisher=»БАСАРАНОВИЧ ЕКАТЕРИНА» pubdate=»2017-01-17″ edition=»ЕВРАЗИЙСКИЙ СОЮЗ УЧЕНЫХ_30.10.16_31(2)» ebook=»yes» ]

